The Five Systems of Dysphagia in COPD
by Jeanna Winchester PhD & Carol Winchester MS SLP CCC
A functioning Respiratory System is critical to sustaining life by bringing essential elements such as oxygen to the body’s cells while removing harmful compounds such as carbon dioxide, simultaneously. There are an average of 480 million microscopic, air-filled structures that are surrounded by a dense network of tiny blood capillaries involved in the exchange of oxygen and carbon dioxide, called alveoli. Alveoli and capillaries are lined with layers of simple squamous epithelium that constitute the respiratory membrane across which oxygen and carbon dioxide can be exchanged between the bloodstream and the outside world. Oxygen and carbon dioxide are pulled in and out of the respiratory tract by expansion and contraction of the lungs (Ferrand, 2014; Samsam, 2015; Shier, Butler & Lewis, 2015).
The primary and accessory muscles of inspiration facilitate contraction of the diaphragm, which pulls the lungs downward and spread out to increase the vertical dimension of the chest cavity, resulting in a change in abdominal pressure. The primary and accessory muscles of inspiration facilitate contraction of the diaphragm, which pulls the lungs downward and spread out to increase the vertical dimension of the chest cavity resulting in a change in abdominal pressure. Abdominal pressure increases and drives the abdomen down and out, which pulls the chest cavity in the transverse dimension. together, the lungs are pulled down and out, which expands the lungs, dropping the intra-lobar pressure and forces air into the alveoli. During passive breathing, the diaphragm relaxes and returns the thorax and lungs to their original shape, increasing intralobar pressure that forces air back out of the body. Overall, the diaphragm moves about a centimeter in either direction during passive breathing. During active breathing, expiration involves the contraction of abdominal muscles that further pushes the diaphragm upward nearly 10cm (Ferrand, 2014; Samsam, 2015; Shier, Butler, Lewis, 2015). These muscles are recruited during conscious, active breathing, and in the case of chronic respiratory diseases, they are recruited to overcome any mechanisms that are inhibiting the natural respiring mechanics in a healthy aging adult.
The natural respiring mechanics of the healthy aging adult are important to the movement of the chest wall and chest cavity, in order for air to enter the lungs. Chest wall compliance, then, is defined as the dimension of change that occurs during respiration. During healthy adult respiration, the lungs and chest wall are like two springs that are pulling against each other, where the chest wall is pulling the thoracic cavity more open, while the lungs try to collapse it. These two structures are dynamically opposing each other and contribute to the elasticity of the respiratory cavity, facilitating respiration without requiring the extra effort provided by the muscular system (Ferrand, 2014; Samsam, 2015; Shier, Butler & Lewis, 2015).
If the lung and chest wall relationship is deleteriously affected, then the lungs will collapse to a degree and the thoracic cavity will expand to a volume larger than the healthy resting volume. Under healthy conditions, the relationship of the chest wall and lung forces are relatively equal, but in dysfunctional conditions, impeded chest wall compliance or non-compliance can have widespread effects on ventilation. Naturally, chest wall compliance, ventilation and the efficiency of the gas exchange are all affected by the changes to the body due to age (Ferrand, 2014; Samsam, 2015; Shier, Butler & Lewis, 2015).
The aging Respiratory System is characterized by a progressive reduction in physiologic capacity, changes in respiratory mechanics, increased rigidity of the chest wall, a more convex thoracic shape, increased ossification and calcification of the costal cartilages, decreased strength of the respiratory muscles, loss of alveolar surface tension, reduced pulmonary capillary blood volume and decreased diameter of the small airways beginning in the third decade of life. Any and all of these age-related changes can cause reduced chest wall compliance, diminished elastic recoil pressures, reductions in vital capacity and an increase in the volume of air remaining in the lungs that does not get utilized during gas exchange. Airway clearance mechanisms are less effective in older individuals, as well. Impaired respiratory mechanics and weakened respiratory muscles can decrease cough effectiveness. All of these factors place elderly persons at greater risk for developing pneumonia with any respiratory illnesses (Fragoso, 2015; Ferrand, 2014; Samsam, 2015; Shier, Butler & Lewis, 2015; Winchester & Winchester, 2015).
Lung diseases are grouped according to how they affect the lungs, particularly if they limit or block airflow or if they affect the gas exchange. Obstructive airway diseases are lung diseases where airflow is interrupted and patients may experience dyspnea (defined as the perceived, subjective, discomfort that occurs with respiratory problems that can range from mild to extreme, and can occur when there are high concentrations of carbon dioxide in the bloodstream (Ferrand, 2014; Samsam, 2015; Shier, Butler, Lewis, 2015).
It is predicted that Chronic Obstructive Pulmonary Disease (COPD) will become the fourth leading cause of death worldwide within the next few years, accounting for more than 145,000 deaths and afflicting more than 24 million Americans. COPD is preventable and treatable, but is not necessarily reversible. Airflow limitations caused by noxious particles or gases, such as cigarette smoke, progressively worsen, often despite treatment. COPD diagnoses account for the greatest number of patients receiving oxygen therapy, currently. Though COPD is primarily a degenerative disease of the respiratory system, classified into 4 stages, it causes systemic breakdown throughout the body. Obstructive respiratory issues, in general, are those that cause blockage of the airways that correspond with smooth airway muscle spasms and often affect exhalation (Ferrand, 2014; Samsam, 2015; Shier, Butler, Lewis, 2015).
The term, COPD, represents the closely related diseases of Emphysema and Chronic Bronchitis, with many patients having Emphysema and Chronic Bronchitis co-morbid diagnoses. In this degenerative respiratory disease, airflow is chronically obstructed, the alveoli are hyperinflated and mucus is secreted in large amounts; along with emphysematous destruction of alveolar walls (impairing gas exchange) and a loss of elasticity in the respiratory tract from lung expansion to alveolar function (Beachy, 2013; Ferrand, 2014; Kakmarek, 2013; Samsam, 2015; Shier, Butler, Lewis, 2015).
Patients often experience cough, phlegm, wheezing, diminishing breath sounds and a progressively slow development of dyspnea that occurs in the later stages of COPD that can sometimes require the need for artificial supply of oxygen and is frequently associated with a reduction in functional ADL status and QOL. As the disease progresses, and hyperinflation ensues, patients may develop barrel chest. When combined with diaphragm flattening and a dimpling inward of the chest wall at the level of the diaphragm on inspiration, a patient may use the accessory muscles of inspiration to draw a breath (instead of using the primary muscles) and develop edema from an abnormal enlargement of the right side of the heart (Beachy, 2013; Ferrand, 2014; Kakmarek, 2013; Samsam, 2015; Shier, Butler, Lewis, 2015).
Emphysema patients are also characterized by a large barrel-shaped chest, a poor air pumping system and in the advanced stages, every single breath can be difficult. Diaphragmatic hyperinflation is noted in this population, as well as in patients with COPD; more oxygen consumption is needed in order to work the respiratory muscles during inhalation/exhalation which comes at a high oxygen cost.
The increased oxygen cost contributes to patients’ limited physical activity and may contribute to the difficulty in weaning patients from mechanical ventilation (Ferrand, 2014; Samsam, 2015; Shier, Butler & Lewis, 2015).
In Chronic Bronchitis, defined as the presence of chronic or recurrent cough that has lasted for 3 consecutive months of the year over the course of 2 consecutive years (Steidl et al., 2014), the bronchial tree is under attack and airflow is obstructed due to inflammation that cases an excess production of mucus and edema. In a healthy individual, approximately 0.1L of mucous is secreted per day, but this increases significantly in the case of Chronic Bronchitis. Additionally, the mucosa becomes thicker and sticky, further impairing ciliary function, blocking or impeding airways that cause air to become stagnant and a breeding ground for bacteria, viruses and pollutants (Ferrand, 2014; Samsam, 2015; Shier, Butler & Lewis, 2015).
In general, patients suffering from Chronic Bronchitis have difficulty with clearing the mucus and the extra effort needed to expel the mucus can scar bronchial tube epithelium. If the mucus is not cleared, there exists a breeding ground for bacteria in the lower airways and an increased the risk of further infection. In Chronic Bronchitis, women are diagnosed more frequently than men (in contrast to COPD, which is the opposite). Similar to COPD and Emphysema, Chronic Bronchitis is primarily caused by the inhalation of cigarette smoke. The degree of dyspnea in Chronic Bronchitis depends on the degree of congestion, the degree of bronchial inflammation, the amount of bronchial mucus secretion and the degree to which the airways are obstructed (Beachy, 2013; Ferrand, 2014; Kakmarek, 2013; Samsam, 2015; Shier, Butler, Lewis, 2015).
Respiratory Dysphagia occurs in COPD as well as its subdivisions of Emphysema and Chronic Bronchitis because there is a loss of the coordination of the coordination of respiration and the functional swallow. Respiratory dysfunction and swallowing dysfunction have been noted in a wide variety of research and clinical settings, and research has shown a close relationship of Respiratory Dysphagia with repeated hospitalization, particularly in the elderly. As many as two-thirds of patients with pneumonia have dysphagia and upwards of 80% of patients readmitted to hospitals with dysphagia have aspiration pneumonia (Cabre et al., 2103).
Complications due to Respiratory Dysphagia in these populations can cause malnutrition, dehydration, aspiration, pneumonia, decreased WOL and mortality. Penetration of the laryngeal vestibule and aspiration of a bolus are common in patients with COPD, because COPD patients have noted delayed closure of the laryngeal vestibule, resulting in respiratory dysfunction and decreased patient safety during eating. COPD patients may breath at points during the respiration cycle that increase the likelihood of aspiration and complications due to COPD may result in increased residue in the oral, pharyngeal and laryngeal cavities. Reduced elevation after a COPD patient has attempted to swallow, along with cricopharyngeal dysfunction, increased needs for swallowing compensatory maneuvers and an increased duration of pharyngeal transit, which may be related to insufficient glottal pressure because patients have reduced expiratory flow. Not surprisingly, COPD patients are noted to have reduced volumes of meal consumption and dysphagia, here, likely contributes to the significant weight loss observed in patients with COPD and its related sieges (Almirall et al., 2012, 2016a & 2016b; Cassani et al., 2015; Steidl et al., 2014; Scelza et al., 2015).
Thus, this literature review demonstrates the importance of effectively managing The Five Systems of Dyspahgia in COPD patients. However, effectively managing the The Five Systems of Dysphagia is a complicated task that necessitates the interaction of the entire interdisciplinary healthcare team. Previous literature has demonstrated the beneficial effect of incorporating instrumentation in the evaluation of The Five Systems of Dysphagia, including the respiratory system, in patients at a high risk for aspiration (Winchester & Winchester, 2015); such as COPD patients who may be experiencing dysphagia.
To that end, the present retrospective investigation sought to evaluate The Five Systems of Dysphagia in patients suffering from COPD, from the perspective of The Five Systems of Dysphagia, utilizing the Dysphagia Systems Test (DST) with endoscopic instrumentation. Dysphagia Management Services LLC (DMS) contracts with more than 1,500 facilities in 32 states across the United States, providing comprehensive evaluations with the DST, that utilizes transnasal fiberoptic endoscopic procedure to assess the functional swallow in patients with possible dysphagia. DMS provided access to all patients assessed over the course of 2014, from the perspective of The Five Systems of Dysphagia, utilizing the DST and transnasal fiberoptic endoscopic instrumentation. Patients with a primary or co-morbid diagnosis of COPD and suspected or noted dysphagia were included (N = 35).
Variables from the DST included in the present pilot retrospective analyses included a change in Food or Liquid Consistency post-DST, an Extended Oral Phase due to extended mastication, oral preparation or bolus transit, a noted Delayed or Intermittent Cough Reflex, observed Delayed Laryngeal Closure, observed Delayed Swallow Reflex Trigger, the presence of Secretions or Residue in the oral, pharyngeal or laryngeal cavities, Premature Spillage into the Hypopharynx, Penetration of the Laryngeal Vestibule, Coughing/Choking during the DST, the presence of Reflux Dysphagia, Aspiration or Silent Aspiration during the DST and the presence of a Hospital Admission during the most recent 3mos to the DST+endoscopic evaluation.
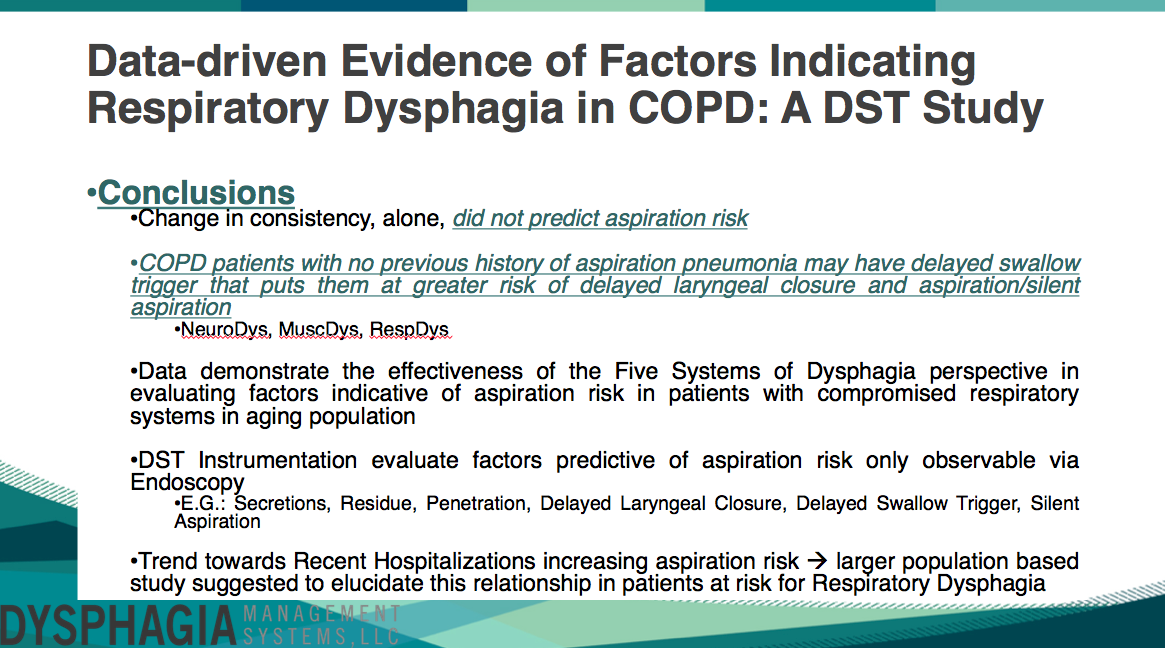
Chi Square analyses indicated that utilizing a change in food consistency, alone, was not predictive of the presence of dysphagia signs or aspiration risk in patients with COPD. Overall, the COPD population did not experience a change in their food consistency (71.4% of patients), with 22.9% of patients having a downgrade in food consistency while only 5.7% of patients had an upgrade in food consistency. However, aspiration occurred in 57.1% of COPD patients.
Other signs of dysphagia that were observable utilizing endoscopy during the DST from the perspective of the Five Systems of Dysphagia include Secretions that were significantly related to the presence of aspiration during the DST (z = 2.42, p = 0.01), Residue in COPD patients was related to the likelihood for penetration (z = 2.56, p = 0.03) and aspiration during the DST (z = 2.56, p = 0.02). Additionally, Penetration was significant related to the likelihood for aspiration during the DST (z = 3.49, p < 0.001); these results are consistent with previous literature.
Further, COPD patients that did not have a documented history of aspiration pneumonia demonstrated the presence of a delayed swallow trigger (z = 2.6, p = 0.2). Dangerously, a Delayed Swallow Trigger was significantly related to the presence of a Delayed Laryngeal Closure during the swallow (z = 2.54, p = 0.02), a Delayed Laryngeal Closure during the swallow was significantly related to the likelihood of Silently Aspirating during the DST (z = 2.38, p = 0.03) and if a patient experienced Silent Aspiration during the DST, they were highly likely to have their Liquid Consistency downgraded (X^2 = 8.8, p = 0.02); representing the presence of Respiratory, Neurological and Muscular Dysphagia in COPD patients.
There were also some non-significant trends, that may become significant if the study was conducted in a greater population of patients. Such as the presence of Premature Spillage and Choking/Coughing during the DST being related to the likelihood of having a Hospitalization within the most recent 3mos to the evaluation (z = 2.1, p = 0.07 and z = 2.09, p = 0.07, respectively).
This study concluded that a change in consistency, alone, did not predict aspiration risk. COPD patients with no previous history of aspiration pneumonia may have Delayed Swallow Triggers that put them at greater risk of Delayed Laryngeal Closure and Aspiration/Silent Aspiration spanning the Neurological, Respiratory and Muscular Systems of Dysphagia. Data also demonstrate the effectiveness of the Five Systems of Dysphagia perspective in evaluating factors indicative of aspiration risk in patients with compromised respiratory systems, particularly in COPD. DST instrumentation evaluates factors predictive of aspiration risk only observable via endoscopy, such as Secretions, Residue, Penetration, Delayed Laryngeal Closure, Delayed Swallow Trigger and Silent Aspiration. Trends also indicated that the presence of a recent hospitalization increases aspiration risk and a larger population based study may elucidate this relationship in patients at risk for Respiratory Dysphagia, further.
REFERENCES:
- Almirall et al., (2012) Complications of oropharyngeal dysphagia: Aspiration pneumonia. Nestle Nutr Inst Workshop Ser. 72: 67-76.Dysphagia. 13: 69-81.
- Almirall et al., (2016) Risk factors for community-acquired pneumonia in adults: A review. Clinical Pulmonary Medicine. 23:99-104. (
- Almirall et al., (2016) Risk factors for community-acquired pneumonia in adults: Recommendations for its prevention. Community Acquired Infection. 2: 32-37.
- Beachey (2013) Respiratory Care Anatomy and Physiology: Foundations for Clinical Practice. Elsevier. 3ed.
- Cabre M, Serra-Prat M, Force L., Almirall J, Palomera E, Calve P (2013) Oropharyngeal dysphagia is a risk factor for readmission for pneumonia in the very elderly persons: Observational prospective study. J Gerontol A Biol Sci Med Sci. doi: 10.1093/Gerona/glt099
- Cassiani RA, Santos CM, Baddini-Martinez J, Dantas RO (2015) Oral and Pharyngeal Bolus Transit in Patients with Chronic Obstructive Pulmonary Disease. International Journal of COPD. 10: 489-496.
- Kackmarek RM, Stoller JK, Heuer AJ (2013) Egan’s Fundamentals of Respiratory Care. Elsevier. 10ed.
- Steidl E, Ribeiro CS, Goncalves BF, Fernandes N, Antunes V, Mancopes R (2015) Relationship between Dysphagia and Exacerbations in Chronic Obstructive Pulmonary Disease: A Literature Review. Internation Archives of Otorhinolaryngology. 19: 074-079.
- Scelza L, Greco CSS, Lopes AJ, Lopes de Melo P (2014) Dysphagia in chronic obstructive pulmonary disease. InTech. pp201-227.
- Winchester J, Winchester C (2015) Cognitive Dysphagia and Effectively Managing the Five Systems. Perspectives in Gerontology. 20:116.